Francisco Church is a rehabilitation specialist and the chief editor of Recovery Ranger. He creates this site to offer guidance and support to individuals seeking...Read more
Alcohol and water are two of the most important elements for life. But did you know that some types of alcohol are more soluble in water than others? In this article, we will explore which alcohol is most soluble in water and why this is important. So, let’s dive in and find out the answer to this intriguing question!
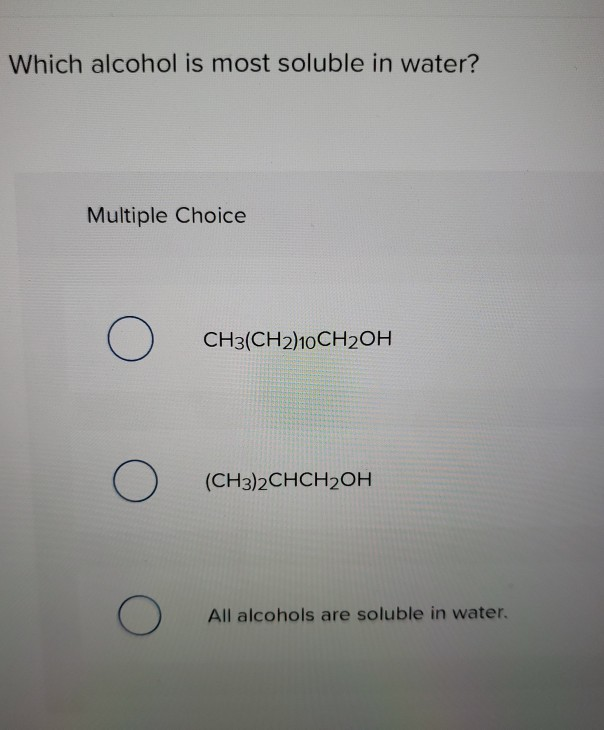
Ethanol is most soluble in water compared to other alcohols. Ethanol has a solubility of 4 g per 100 g of water at 20 °C, and the solubility increases slightly with increasing temperature. Ethanol is a polar molecule due to the lone pair of electrons on the oxygen atom, making it soluble in polar solvents such as water. Other alcohols such as methanol and isopropanol are also soluble in water. The solubility of these other alcohols is lower than that of ethanol.
Contents
- Types of Alcohol and Their Solubility in Water
- Other Types of Alcohols and Their Solubility in Water
- Higher Alcohols and Their Solubility in Water
- Top 6 Frequently Asked Questions
- What is Solubility?
- What Alcohol is Most Soluble in Water?
- What Factors Affect Ethanol Solubility?
- Why is Ethanol Soluble in Water?
- What are the Uses of Ethanol Solubility?
- What is the Solubility of Other Alcohols in Water?
- Ranking solubility of organic compounds in water based on IMF
- Is Diethylpropion A Stimulant?
- Is Alcohol A Inflammatory?
- Does Alcohol Make A Uti Worse?
Types of Alcohol and Their Solubility in Water
Alcohols are organic compounds that have a hydroxyl group (-OH) attached to a carbon atom. Depending on the type of alcohol, their solubility in water varies. In general, the more hydroxyl groups an alcohol has, the more soluble it is in water.
Methanol
Methanol, also known as methyl alcohol, is an alcohol with a single hydroxyl group. It is very soluble in water, with a solubility of about 65 grams per 100 milliliters of water at room temperature. It is also very volatile, meaning that it evaporates quickly from the liquid to the gas phase.
Methanol is toxic and can cause blindness if ingested. It is not suitable for human consumption, and is mainly used as an industrial solvent and a fuel.
Ethanol
Ethanol, or ethyl alcohol, is an alcohol with two hydroxyl groups. It is the type of alcohol found in alcoholic beverages. It is more soluble in water than methanol, with a solubility of about 88 grams per 100 milliliters of water at room temperature. It is also less volatile than methanol, meaning that it evaporates more slowly from the liquid to the gas phase.
Ethanol is a common solvent for laboratory and industrial applications, and is also suitable for human consumption. It has been used as an intoxicating beverage for centuries, and is the main ingredient in many alcoholic drinks.
Other Types of Alcohols and Their Solubility in Water
Glycerol
Glycerol, or glycerin, is an alcohol with three hydroxyl groups. It is very soluble in water, with a solubility of about 92 grams per 100 milliliters of water at room temperature. It is also very viscous and sweet-tasting, which makes it a popular ingredient in many food products.
Glycerol is used as an emulsifier, humectant, and sweetener in food products, as well as a solvent in medical and pharmaceutical products. It is also used in the manufacture of explosives and nitroglycerin.
Propanol
Propanol, or propyl alcohol, is an alcohol with four hydroxyl groups. It is slightly less soluble in water than glycerol, with a solubility of about 70 grams per 100 milliliters of water at room temperature. It is also less viscous than glycerol, and has a higher boiling point.
Propanol is a common industrial solvent and is used as a fuel for some types of fuel cells. It is also used in the manufacture of many chemicals, such as ethylene glycol, acetone, and acetic acid.
Higher Alcohols and Their Solubility in Water
Hexanol
Hexanol, or hexyl alcohol, is an alcohol with six hydroxyl groups. It is slightly less soluble in water than propanol, with a solubility of about 62 grams per 100 milliliters of water at room temperature. It is also less volatile than propanol, meaning that it evaporates more slowly from the liquid to the gas phase.
Hexanol is a common industrial solvent and is used in the manufacture of some chemicals, such as ethylene glycol and acetone. It is also used as a fuel for some types of fuel cells.
Octanol
Octanol, or octyl alcohol, is an alcohol with eight hydroxyl groups. It is less soluble in water than hexanol, with a solubility of about 40 grams per 100 milliliters of water at room temperature. It is also less volatile than hexanol, meaning that it evaporates more slowly from the liquid to the gas phase.
Octanol is a common industrial solvent and is used in the manufacture of some chemicals, such as ethylene glycol and acetone. It is also used as a fuel for some types of fuel cells.
Top 6 Frequently Asked Questions
What is Solubility?
Solubility is the ability of a substance, usually a solid, to dissolve in a liquid. Solubility can be affected by the temperature, pressure, and the presence of other substances in the solution. Solubility is measured in moles per liter (mol/L). This is the concentration of the solute (the substance being dissolved) in the solvent (the liquid it is being dissolved in).
What Alcohol is Most Soluble in Water?
The alcohol that is most soluble in water is ethanol. Ethanol is the type of alcohol found in alcoholic beverages, and it is the most water-soluble of all the common alcohols. Ethanol is soluble in water in all proportions, and it is miscible (can be mixed evenly) in all proportions. Other common alcohols such as methanol and isopropanol are also miscible in water, but they are less soluble than ethanol.
What Factors Affect Ethanol Solubility?
Several factors affect the solubility of ethanol in water. These include temperature, pressure, and the presence of other substances. As temperature increases, the solubility of ethanol in water decreases. The same is true for pressure: as pressure increases, the solubility of ethanol in water decreases. Also, the presence of other substances, such as salts or sugars, can affect the solubility of ethanol in water.
Why is Ethanol Soluble in Water?
Ethanol is soluble in water because of its polar nature. Ethanol molecules have a polar head and a non-polar tail, which makes them attracted to water molecules. This attraction allows ethanol molecules to dissolve in water.
What are the Uses of Ethanol Solubility?
Ethanol solubility is important in many industries and applications. In the food and beverage industry, ethanol is used to dissolve flavors, colors, and other additives. In the pharmaceutical industry, ethanol is used to dissolve active ingredients in medications. In the fuel industry, ethanol is used to produce biofuels.
What is the Solubility of Other Alcohols in Water?
The solubility of other alcohols in water varies. Methanol and Isopropanol are also miscible in water, but they are less soluble than ethanol. Propanol and Butanol are slightly soluble in water, while higher alcohols such as Pentanol and Hexanol are practically insoluble in water.
Ranking solubility of organic compounds in water based on IMF
In conclusion, alcohol’s solubility in water can vary depending on the type of alcohol. Ethanol is the most soluble alcohol in water and is generally used for a variety of commercial and industrial applications. Propanol and butanol are less soluble in water than ethanol, but still dissolve in it. Finally, methanol is the least soluble of the four alcohols discussed and has limited applications in water.
Francisco Church is a rehabilitation specialist and the chief editor of Recovery Ranger. He creates this site to offer guidance and support to individuals seeking to overcome addiction and achieve lasting sobriety. With extensive experience in the field of addiction treatment, Francisco is dedicated to helping individuals access the resources they need for successful recovery.
- Latest Posts by Francisco Church
-
Is Diethylpropion A Stimulant?
- -
Is Alcohol A Inflammatory?
- -
Does Alcohol Make A Uti Worse?
- All Posts