Francisco Church is a rehabilitation specialist and the chief editor of Recovery Ranger. He creates this site to offer guidance and support to individuals seeking...Read more
Alcohol is a widely consumed substance all around the world, and its effects on people are well-known. In this article, we will explore the physical and chemical properties of alcohol and debate whether or not it can be considered a pure substance. So, is alcohol a pure substance? Let’s take a look!
Alcohol is a pure substance that is made up of molecules, which contain only one type of atom. Alcohol is composed of two elements: carbon and hydrogen. It is typically found in alcoholic beverages, such as beer and wine, and can also be produced synthetically. Alcohol is classified as a depressant drug, which means it slows down the body’s functions, including the brain and nervous system.
Alcohol affects each person differently, depending on their body type, weight, and how much they have consumed. It is important to consume alcohol in moderation, as excessive drinking can lead to health issues, such as liver damage, increased risk of cancer, and memory loss.
Contents
What is the definition of a Pure Substance?
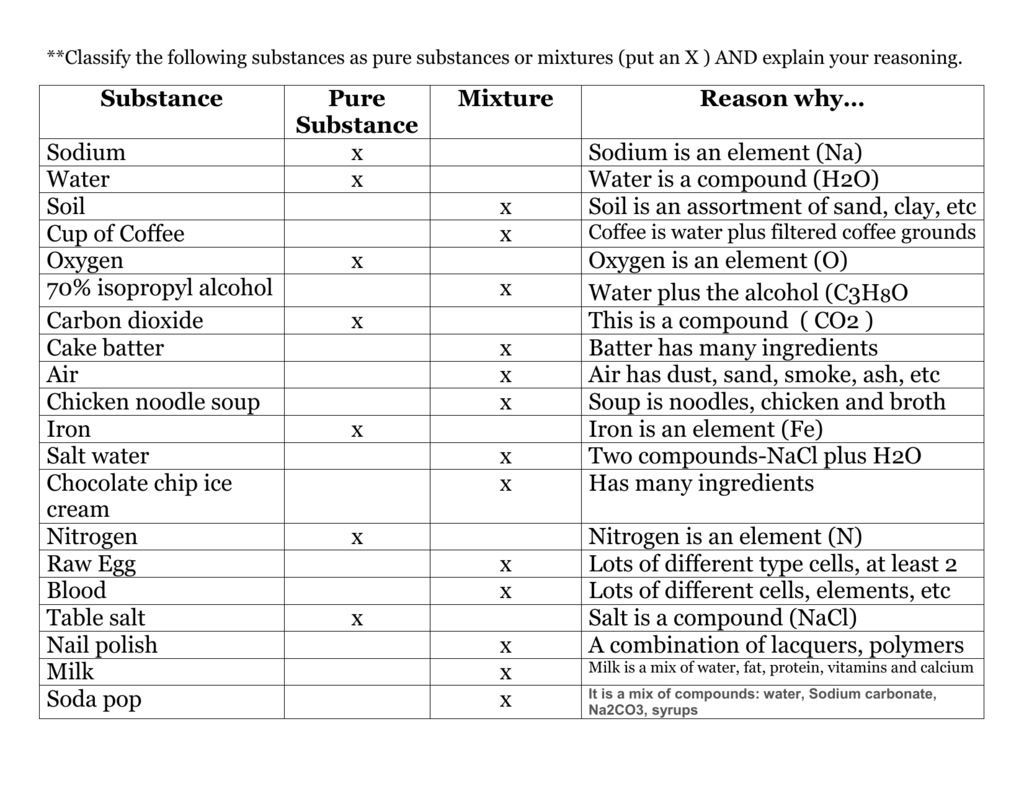
A pure substance is a material that is composed of only one type of atom or molecule. It is a single type of chemical compound, irrespective of the amount of the sample. A pure substance can be either an element or a compound. Examples of pure substances include oxygen, helium, and water.
The distinction between pure substances and mixtures is that pure substances cannot be separated into their components by physical means, such as filtering, centrifuging, or distillation. Mixtures, on the other hand, can be separated into their components. For example, a mixture of salt and water can be separated into its components by evaporation.
Pure substances have unique physical and chemical properties that are not shared by mixtures. For example, water always has the same freezing and boiling points and the same density, regardless of the amount of water present.
Is Alcohol a Pure Substance?
Alcohol is a type of organic compound, and as such it can exist in either a pure or impure form. Pure alcohol is composed of molecules of a single type of alcohol, and is therefore considered a pure substance. Ethanol, for example, is a type of pure alcohol.
The most common form of alcohol, however, is impure alcohol, which is composed of a mixture of different types of alcohol molecules. This impure form of alcohol is also referred to as a distilled spirit, and is the type of alcohol found in alcoholic beverages such as beer, wine, and spirits.
Impure alcohol, or distilled spirits, can be further broken down into different types of mixtures, such as liqueurs, cordials, and aperitifs. These mixtures contain various other ingredients, such as sugar, herbs, and spices, which are not part of the pure alcohol molecule.
What is Ethanol?
Ethanol is a type of pure alcohol molecule and is the type of alcohol found in alcoholic beverages. It is the most commonly used type of alcohol in the world and is the type of alcohol found in beer, wine, and spirits.
Ethanol is a colorless, odorless, and flammable liquid and is produced by the fermentation of sugars by yeast. It is a type of organic compound and is composed of carbon, hydrogen, and oxygen atoms.
Ethanol has a boiling point of 78.3°C (173.14°F) and a melting point of -114°C (-173.2°F), and has a slightly sweet taste.
What is Isopropyl Alcohol?
Isopropyl alcohol, also known as isopropanol, is a type of pure alcohol molecule. It is a colorless, flammable liquid with a slightly sweet odor, and is used in many products such as rubbing alcohol, paint thinners, and antifreeze.
Isopropyl alcohol is produced by the hydration of propylene, and is composed of carbon, hydrogen, and oxygen atoms. It has a boiling point of 82.5°C (180.5°F) and a melting point of -89°C (-128.2°F).
Isopropyl alcohol is a type of volatile organic compound (VOC) and is classified as a hazardous air pollutant by the United States Environmental Protection Agency (EPA).
What is Methyl Alcohol?
Methyl alcohol, also known as methanol, is a type of pure alcohol molecule. It is a colorless, flammable liquid with a slightly sweet odor, and is used in many products such as solvents, antifreeze, and fuel.
Methyl alcohol is produced by the catalytic hydrogenation of carbon monoxide, and is composed of carbon, hydrogen, and oxygen atoms. It has a boiling point of 64.7°C (148.5°F) and a melting point of -97.7°C (-144.1°F).
Methyl alcohol is a type of volatile organic compound (VOC) and is classified as a hazardous air pollutant by the United States Environmental Protection Agency (EPA).
What is Denatured Alcohol?
Denatured alcohol is a type of impure alcohol, or distilled spirit, that is composed of a mixture of different types of alcohol molecules. It is sometimes referred to as “industrial alcohol,” and is used for a variety of purposes such as cleaning agents, paint thinners, and antifreeze.
Denatured alcohol is produced by adding denaturants, such as methanol, to ethanol to make it unfit for human consumption. It is composed of a mixture of different types of alcohol molecules, and has a variety of uses.
Denatured alcohol is a type of volatile organic compound (VOC) and is classified as a hazardous air pollutant by the United States Environmental Protection Agency (EPA).
Few Frequently Asked Questions
What is a Pure Substance?
A pure substance is a single type of material made up of only one kind of atom or molecule. It is composed of only one kind of particle and cannot be broken down into other types of matter. Examples of pure substances are elements such as gold, oxygen, and hydrogen, and compounds such as water, salt, and sugar.
Is Alcohol a Pure Substance?
No, alcohol is not a pure substance. Alcohol is a compound composed of two types of molecules: ethanol and water. Ethanol is an organic molecule made up of hydrogen, oxygen, and carbon atoms, while water is a combination of hydrogen and oxygen molecules. Therefore, alcohol is not considered a pure substance.
What are the Different Types of Alcohol?
There are several different types of alcohol. Ethyl alcohol, or ethanol, is the type of alcohol found in alcoholic beverages. Other types of alcohol include isopropyl alcohol, methanol, and butanol. These types of alcohol are used in industrial and medical applications.
What is the Chemical Formula of Alcohol?
The chemical formula of alcohol is C2H6O. This chemical formula describes ethanol, which is the type of alcohol found in alcoholic beverages. The C2H6O formula stands for two carbon atoms, six hydrogen atoms, and one oxygen atom.
Is Alcohol a Solid, Liquid, or Gas?
Alcohol is a liquid at room temperature. It can be heated to a gas or cooled to a solid, but at room temperature it is a liquid.
How is Alcohol Made?
Alcohol is made through a process called fermentation. This process involves adding yeast to a mixture of sugar and water. The yeast consumes the sugar and produces ethanol, which is the type of alcohol found in alcoholic beverages. The ethanol is then distilled to separate it from the water and other impurities.
Pure Substance vs Mixture
In conclusion, it is clear that alcohol is a complex substance and can be classified as both a pure and an impure substance. While it is composed of a single type of molecule, it also contains impurities, making it an impure substance. It can be both beneficial and harmful depending on its consumption, so it is important to keep this in mind when consuming alcohol. Ultimately, it is up to the individual to decide whether or not to consume alcohol, and to do so responsibly.
Francisco Church is a rehabilitation specialist and the chief editor of Recovery Ranger. He creates this site to offer guidance and support to individuals seeking to overcome addiction and achieve lasting sobriety. With extensive experience in the field of addiction treatment, Francisco is dedicated to helping individuals access the resources they need for successful recovery.
- Latest Posts by Francisco Church
-
Is Diethylpropion A Stimulant?
- -
Is Alcohol A Inflammatory?
- -
Does Alcohol Make A Uti Worse?
- All Posts