Francisco Church is a rehabilitation specialist and the chief editor of Recovery Ranger. He creates this site to offer guidance and support to individuals seeking...Read more
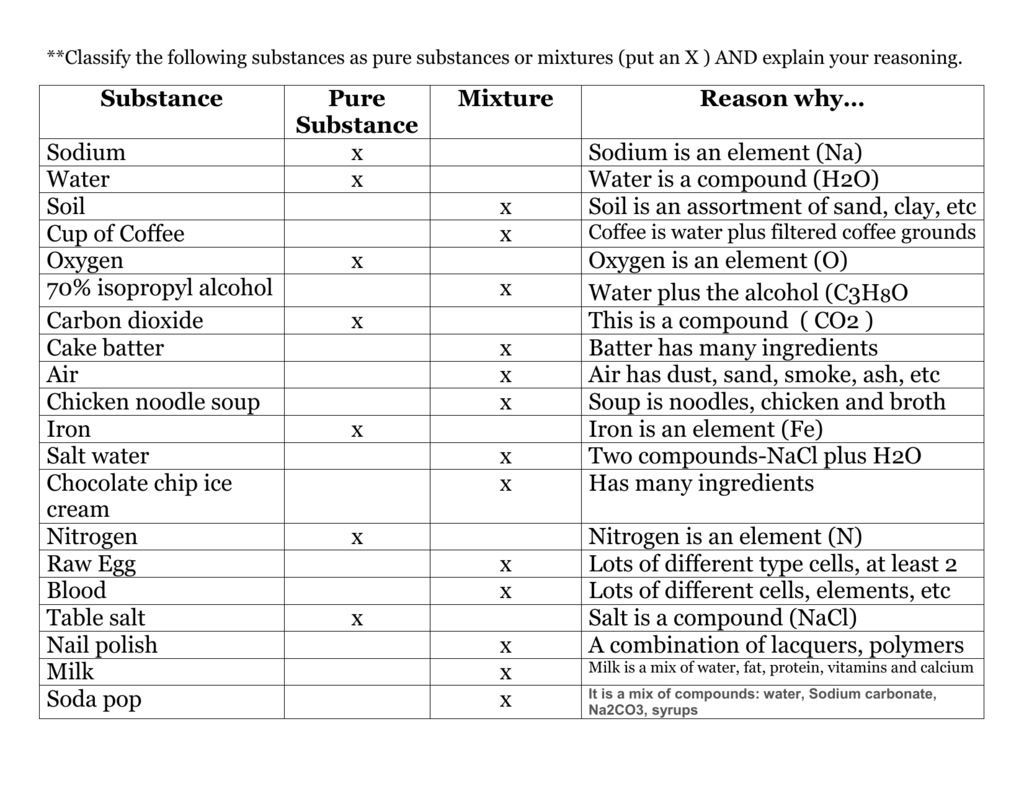
Alcohol is a common substance used in everyday life, but what exactly is it? Is it a pure substance or a mixture? This is a question that has been debated for many years, and one that deserves further exploration. In this article, we will dive into the debate between whether alcohol is a pure substance or a mixture, examining its chemical composition and the implications of each classification. We will also explore the effects of alcohol on the body and its potential risks. By the end of this article, you should have a better understanding of what exactly alcohol is and whether it is a pure substance or a mixture.
Alcohol is a mixture, not a pure substance. It is composed of two or more components that are mixed together, but not chemically combined. Alcohol can be composed of different types of molecules, such as ethanol, methanol, propanol, and butanol. These molecules can be either solids, liquids, or gases, depending on the type of alcohol. Alcohols are found in many everyday items, such as beer, wine, spirits, and even cleaning agents.
Alcohol: A Pure Substance or a Mixture?
Alcohol is an important part of our lives, and it has been for thousands of years. But is it a pure substance or a mixture? This is an important question to answer, as it affects how we handle and interact with alcohol in our day-to-day lives.
To understand the answer to this question, it’s important to understand what a pure substance and a mixture are. A pure substance is a single element or compound that cannot be broken down into other substances. A mixture, on the other hand, is made up of two or more substances that can be separated through physical means.
What is Alcohol?
The most common type of alcohol is ethanol, which is a type of organic compound. This means that it is made up of carbon and hydrogen atoms, with a chemical formula of C2H5OH. Ethanol is a colorless, flammable liquid that is soluble in water and has a boiling point of 78.3°C.
Ethanol can be produced through the fermentation of sugar or starch, or it can be synthesized from ethylene or acetylene. It is often used in alcoholic beverages, as a fuel, or as a solvent. It is also the active ingredient in many products such as mouthwash, hand sanitizer, and even antifreeze.
Is Alcohol a Pure Substance?
The short answer is yes, alcohol is a pure substance. Ethanol is a single compound, and it cannot be broken down into any other substances. This means that ethanol is a pure substance, and it cannot be broken down into any other substances.
However, most alcoholic beverages are not pure ethanol. They are mixtures of ethanol and other substances, such as water, flavorings, and preservatives. These substances can be separated through physical means, so alcoholic beverages are not considered pure substances.
Is Alcohol a Mixture?
Yes, alcoholic beverages are mixtures of ethanol and other substances. Alcoholic beverages are made up of ethanol and other substances, such as water, flavorings, and preservatives. These substances can be separated through physical means, so alcoholic beverages are considered mixtures.
In addition, some alcoholic beverages contain additional ingredients that cannot be separated through physical means. For example, some beers contain additional yeast, which cannot be separated from the mixture. These types of alcoholic beverages are also considered mixtures.
Conclusion
To conclude, alcohol is a pure substance, but alcoholic beverages are mixtures. Ethanol is a single compound, and it cannot be broken down into any other substances. However, alcoholic beverages are mixtures of ethanol and other substances, such as water, flavorings, and preservatives. These substances can be separated through physical means, so alcoholic beverages are not considered pure substances.
Top 6 Frequently Asked Questions
What is a Pure Substance?
A pure substance is a material that is made up of only one type of atom or molecule. It has a unique chemical composition and always has the same properties. Examples of pure substances include elements like oxygen and iron, and compounds like water and sugar.
What is a Mixture?
A mixture is a combination of two or more substances where each substance retains its own chemical properties. The substances may be either pure substances or mixtures themselves. Examples of mixtures include air (a combination of nitrogen, oxygen and other gases) and seawater (a combination of water and salts).
Is Alcohol a Pure Substance or a Mixture?
Alcohol is a mixture. It is typically composed of water and one or more types of alcohol molecules. The most common type of alcohol is ethanol, which is a clear, colorless liquid with a distinctive odor and taste. Other types of alcohols include methanol, isopropyl alcohol, and others.
What Are the Properties of Alcohol?
The properties of alcohol vary depending on the type of alcohol. Generally, alcohols are volatile liquids with strong odors and tastes. They are also flammable and can dissolve in water. Alcohols have a low boiling point, so they evaporate easily.
How Is Alcohol Made?
Alcohol is made by the fermentation of carbohydrates, such as sugars and starches. The fermentation process involves the use of yeast, which converts the carbohydrates into alcohol and carbon dioxide. This process is used to make alcoholic beverages such as beer, wine, and spirits.
What Are the Effects of Alcohol?
Alcohol consumption can have a range of effects on the body, including impaired judgment and coordination, slowed reaction time, and impaired memory. In high doses, alcohol can cause vomiting, unconsciousness, coma, and even death. Long-term alcohol consumption can also lead to liver damage and other health problems.
Pure Substance vs Mixture
In conclusion, alcohol is both a pure substance and a mixture. Its chemical structure is a pure substance, meaning that it is composed of the same type of atoms, but it can also be a mixture when combined with other substances. The purity of alcohol depends on the mixture it is combined with, and this can impact its effects. Alcohol can be both a healthful and dangerous substance, depending on how it is consumed and in what mixture.
Francisco Church is a rehabilitation specialist and the chief editor of Recovery Ranger. He creates this site to offer guidance and support to individuals seeking to overcome addiction and achieve lasting sobriety. With extensive experience in the field of addiction treatment, Francisco is dedicated to helping individuals access the resources they need for successful recovery.
- Latest Posts by Francisco Church
-
Is Diethylpropion A Stimulant?
- -
Is Alcohol A Inflammatory?
- -
Does Alcohol Make A Uti Worse?
- All Posts